Quality Consultation For the Pharmaceutical & Biologics Industries
Audits & Gap Assessments
Mock Inspections
Facility Design Reviews
Internal Audits of Quality Units
Qualified Person (UK Based)
Qualification and Validation Review
Due Diligence for Mergers/Acquisitions
Good Documentation Practices an Data Integrity
Training, Coaching and Mentoring
Technical Support & Technical Writing
Why Jeff Yuen & Associates, Inc.?
It is important to ensure that client engagements are properly executed and that customers remain satisfied at all times. JYA is not afraid to "course correct" whenever needed. It is also important to emphasize that JYA does not promote or accept assignments where we cannot bring the proper subject matter experts to the client.

Why Jeff Yuen & Associates, Inc.?
It is important to ensure that client engagements are properly executed and that customers remain satisfied at all times. JYA is not afraid to "course correct" whenever needed. It is also important to emphasize that JYA does not promote or accept assignments where we cannot bring the proper subject matter experts to the client.
Industry and Health Authority Experience


Audits and mock inspections prior to an FDA PAI or PLI inspection are critical to understanding risks and managing compliance risks. Proper Sponsor oversight is mandatory from a regulatory compliance and business continuity perspective. JYA provides audit support particularly where and when an outsourced model makes sense. Dependent on the “client’s” procedures, we are capable of scheduling and performing audits including review of acceptability of audit responses. Our goal is to keep senior management informed of deficiencies and/or potential regulatory findings (FDA 483s or further regulatory actions: Warning Letters, etc.) and the suitability and timeliness of CAPAs taken to address gaps or deficiencies after completion of the audit. NOTE: JYA covers all GXP areas: GLP, GCP, GMP, Combo Products, etc.
Services We Offer
Audits and Mock Inspections
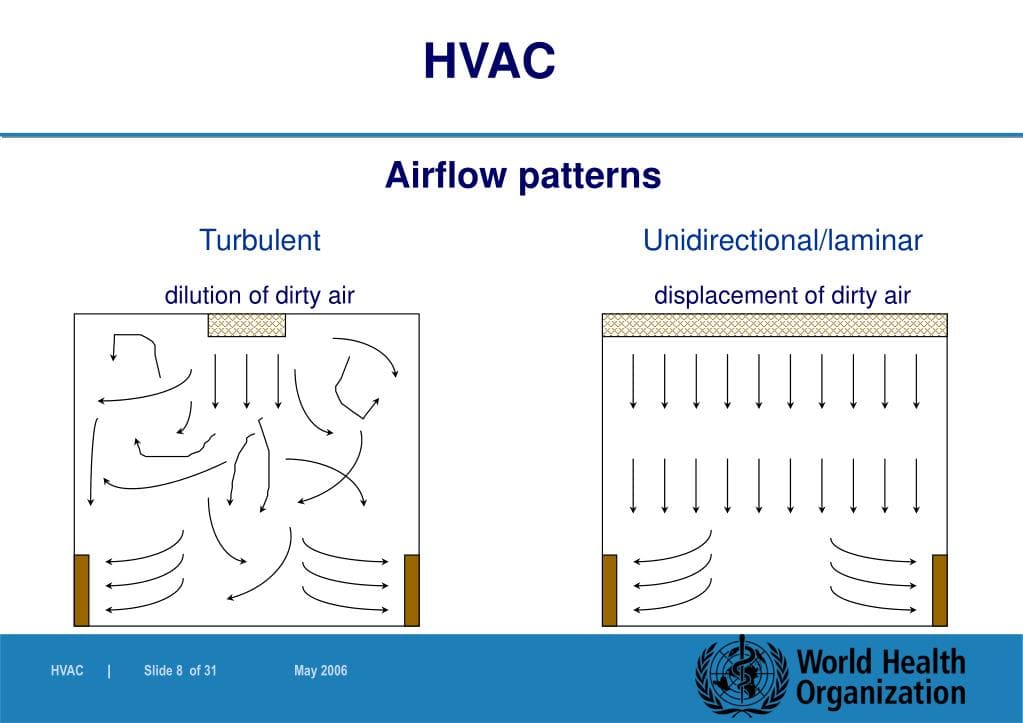
Air Flow Visualization
Drug Substance / API
Cell and Gene Therapy
GLP / GCP/ BIMO/ Data Integrity (DI)
ISO/ Medical Devices / Combination Products
Computer Software Validation (CSV) / Data Integrity (DI) / Part 11 Compliance / ALCOA
Compounding Pharmacies (503A / 503B)
Drug Substance / API
Expert Witness
Validation, Commissioning and Qualification
Project Management
SOP / Technical Writing / Documentation Management
Services We Offer
Auditing And Remediation
Small Molecule
Finished
Biologics, C/G Therapy, Viral Products, CRISPR
GLP And GCP
(BIMO)
Medical Devices Combination Products
Data Integrity/Part 11 and Computer Validation
Compounding Pharmacies (503a and b)
Drug Substance/Active Pharmaceutical Ingredients, Critical Starting Materials, Excipients
Expert Witness
Validation, Commissioning and Qualification
Project Management
Cosmetics Dietary Supplements and Neutraceuticals
We’re Delivering the Best Consulting & Finance Services
Testimonials





New Case Studies
Finance Consulting
Substantial Business
Business Planning
Who We Are
FDA’s Pacific Regional Biotech Team (pre-cursor to ORA Team Biologics)
Foreign Inspection Cadre
Los Angeles District Pre-Approval Manager and Drug Team Leader
of Experience
of Experience