Air Flow Visualization
Contact us to the email
Mail Anytime
support@jeffyuen.com
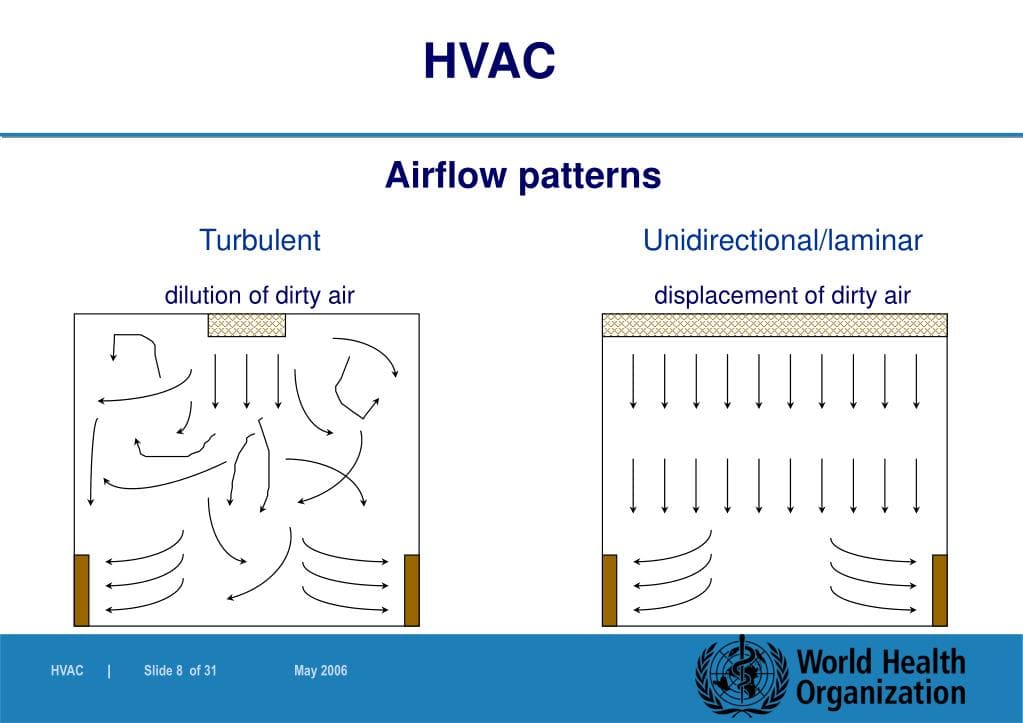
The science of rendering airflow patterns visible to allow analysis of flow is termed “Airflow Visualization Studies” (i.e., smoke studies). Airflow visualization studies are not just a regulatory requirement, they provide critical data and pertinent information necessary to properly support and defend your aseptic operations from an overall contamination control strategy (CCS) particularly regarding the demonstration of proper aseptic techniques and behaviors which remain closely scrutinized by regulatory agency inspection from a sterility assurance perspective.
Proper and effective demonstration of airflow patterns allow multiple cross-functional disciplines to establish a baseline (validation); continuously monitor; actively optimize or evaluate engineering controls; and investigate (as needed) proper or improper aseptic operator techniques and behaviors not only in the critical ISO 5 aseptic processing zones but also while aseptic operator’s transition:
- From ISO 5 or 6 background to critical ISO 5 aseptic filling zones (traditional RABS filling lines and aseptic processing areas (APAs);
- From ISO 7 to ISO 5 (isolator filling lines during pre-VHP open door set up interventions);
- From ISO 7 to ISO 5 (cell and gene therapy / inoculum preparation and/or bioburden reduced DS filling), etc.
Human operators remain the primary source of contamination and risk to sterility assurance.
Key reasons and/or emphasis points from a CGMP compliance perspective are noted as follows:
- Smoke study data can be used by the engineering group to ensure that “unidirectional” airflow patterns are maintained in the critical Grade A / ISO 5 aseptic processing zones.
- Smoke studies are used to demonstrate that proper “first air” principles at the critical height across aseptic filling, stoppering and crimping operations during static and simulated dynamic aseptic operations.
- Smoke studies provide key training materials for onboarding of new aseptic operators and refresher aseptic operator training QA, Micro and Production personnel should review media fill and smoke study videos to ensure proper consistency of aseptic techniques and behaviors.
- Smoke studies in conjunction with proper I-REM and EM Risk Assessments are critically important when it comes to establishing a proper EM program from a site selection and sample collection perspective.
At Jeff Yuen & Associates, Inc. our experts have decades of experience in assessing the results of the studies, recommending adjustments to both engineering controls, aseptic operator (person-in-plant) real time coaching on proper aseptic techniques and behaviors, and preparation of FDA 483 and Warning Letter responses needed to address smoke study deficiencies.
Our team has worked collaboratively with our clients to properly present smoke study videos and reports. We bring a unique blend of former FDA and industry experience and expertise to the development of your smoke studies to ensure that they meet or exceed current industry and FDA expectations. Our team has an excellent track record of working with and coaching client SMES through their smoke study presentations and discussions with investigators / inspectors during high profile regulatory inspections.
Our unique strategy for airflow simulation includes:
- Expertise in protocol development that is customized to your facility and operations
- On-site hands-on training prior to execution
- Selection of appropriate calibrated and qualified equipment from our approved equipment list
- Use of our custom blend of tracer particles to allow complete visualization of flow
- Use of appropriate lighting and high-resolution camera angles to ensure complete visualization
- Comprehensive assessment of engineering controls, operating ranges, and process behaviors
- Assessment and expert recommendations for areas evaluated
After execution of the protocol and data analysis, a final report will be provided which will assess all data and information against the pre-determined acceptance criteria and will provide the narrative needed to 1.) make necessary internal improvements to engineering controls and aseptic behavior and 2.) provide to regulatory agencies during review or inspection.
Our dedicated team will also be available to answer any questions regarding airflow visualization studies and will coach and train your internal engineers upon requested and/or as needed.