SERVICES
We offer a comprehensive service offering to meet your drug or device compliance needs
Services We Offer
Audits and Mock Inspections
You are the priority and the associates at Jeff Yuen & Associates work to ensure...
Validation, Commissioning and Qualification
YA is well versed in the regulations and requirements for facility design & construction...
Executive Quality Leadership / Mentorship and Management Support
JYA continues to provide executive leadership and mentorship to key site leaders in the areas...
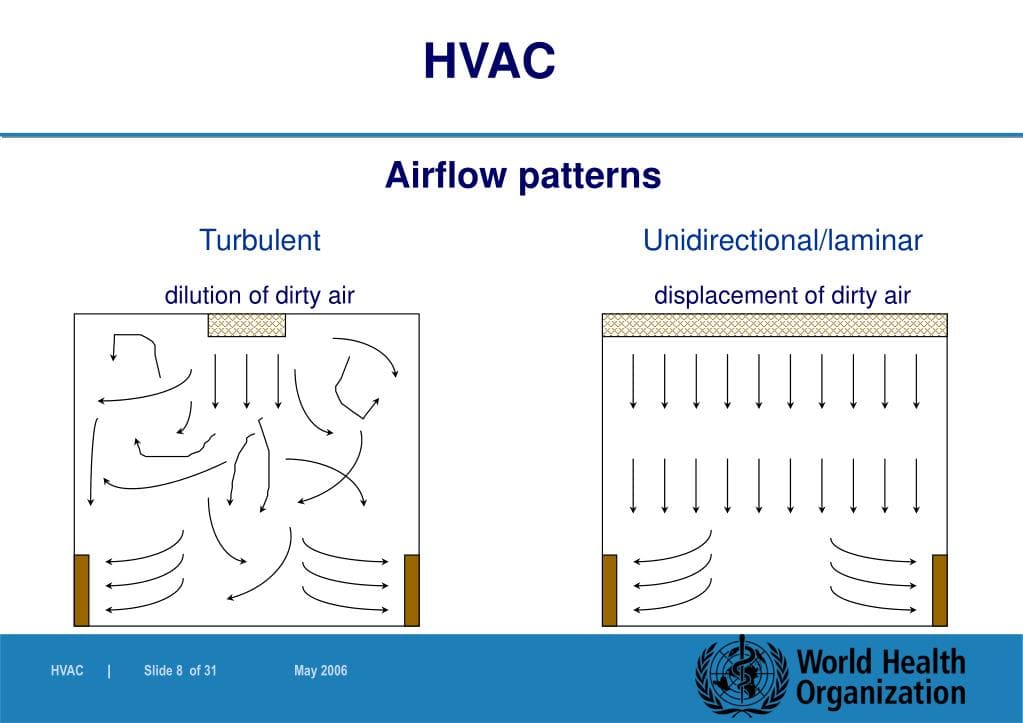
Air Flow Visualization
The science of rendering airflow patterns visible to allow analysis of flow is termed “Airflow Visualization Studies” (i.e., smoke studies)....
Regulatory Affairs
The JYA team leverages significant regulatory experience to provide the expert regulatory....
Qualified Person (QP) Services
For the European markets, QPs are required to perform qualification audits of sites producing...
Project Management
We can manage the project for you. We have real world experience managing...
Expert Witness
No one wants to be in the situation to need an expert witness, but when you do need one...
Welcome
The Real World Experience
The right consultation on right time can keep your business to keep growing.
- Sterility Assurance and CMC Reviews
- Expert Witness
- Vendor/Supplier Qualification Audits
Consulting
Consulting
60%
30
+
Industry Experience Years
2000
+
Cumulative Industry Years
20
+
Staff Health Authority Average Years
350
Cumulative Health Authority Years
Portfolio of Services
Our team at Jeff Yuen & Associates, Inc provides the highest quality service for all pharmaceutical phases including pre-clinical, clinical and commercial operations, testing and regulatory support.
Qualification and Validation Review
Audits & Gap Assessments
Internal Audits of Quality Units
Facility Design Reviews
Due Diligence for Mergers/Acquisitions
Qualified Person (UK Based)
Vendor/Supplier Qualification Audits
Technical Support & Technical Writing
Training, Coaching and Mentoring
Good Documentation Practices an Data Integrity
Back Room Inspection Management or Support
Data Integrity/Part 11 Assessments and Computer System Validation
Mock Inspections
Regulatory & CMC Support (CDER/CBER)
Sterility Assurance and CMC Reviews
Get to Know About Company
CDR Jeff Yuen is a well-respected domestic and international speaker with over 32 years of compliance and regulatory experience; Jeff was a peace officer/investigator with the State of California, Food and Drug Branch and a Consumer Safety Officer for the US FDA. During his tenure with the US FDA, he was rapidly promoted to the rank of Commander in the US Public Health Service.
Jeff Yuen
- Founder
- FDA’s Pacific Regional Biotech Team
- Los Angeles District Pre-Approval
- Foreign Inspection Cadre.